Abstract
The integrity of the mammalian epidermis depends on a balance of proliferation and differentiation in the resident population of stem cells1.The kinase RIPK4 and the transcription factor IRF6 are mutated in severe developmental syndromes in humans, and mice lacking these genes display epidermal hyperproliferation and soft-tissue fusions that result in neonatal lethality2,3,4,5.我们对这些基因如何控制epi的理解dermal differentiation is incomplete. Here we show that the role of RIPK4 in mouse development requires its kinase activity; that RIPK4 and IRF6 expressed in the epidermis regulate the same biological processes; and that the phosphorylation of IRF6 at Ser413 and Ser424 primes IRF6 for activation. Using RNA sequencing (RNA-seq), histone chromatin immunoprecipitation followed by sequencing (ChIP–seq) and assay for transposase-accessible chromatin using sequencing (ATAC-seq) of skin in wild-type and IRF6-deficient mouse embryos, we define the transcriptional programs that are regulated by IRF6 during epidermal differentiation. IRF6 was enriched at bivalent promoters, and IRF6 deficiency caused defective expression of genes that are involved in the metabolism of lipids and the formation of tight junctions. Accordingly, the lipid composition of the stratum corneum ofIrf6−/−skin was abnormal, culminating in a severe defect in the function of the epidermal barrier. Collectively, our results explain how RIPK4 and IRF6 function to ensure the integrity of the epidermis and provide mechanistic insights into why developmental syndromes that are characterized by orofacial, skin and genital abnormalities result when this axis goes awry.
This is a preview of subscription content,access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 per month
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Get just this article for as long as you need it
$39.95
Prices may be subject to local taxes which are calculated during checkout
Data availability
The data sets generated in this study are available from the corresponding authors on reasonable request. Gel source data can be found in Supplementary Fig.1.Source data for all graphs are provided.
Code availability
All custom code can be accessed athttps://github.com/rreja/Oberbeck_Nature_2019.
References
福克斯,大肠皮肤干细胞:上升到水面。J. Cell Biol.180, 273–284 (2008).
Holland, P. et al. RIP4 is an ankyrin repeat-containing kinase essential for keratinocyte differentiation.Curr. Biol.12, 1424–1428 (2002).
Ingraham, C. R. et al. Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6).Nat. Genet.38, 1335–1340 (2006).
Kondo, S. et al. Mutations inIRF6cause Van der Woude and popliteal pterygium syndromes.Nat. Genet.32, 285–289 (2002).
Mitchell, K. et al. Exome sequence identifiesRIPK4as the Bartsocas–Papas syndrome locus.Am. J. Hum. Genet.90, 69–75 (2012).
Rountree, R. B. et al. RIP4 regulates epidermal differentiation and cutaneous inflammation.J. Invest. Dermatol.130, 102–112 (2010).
De Groote, P. et al. A novel RIPK4–IRF6 connection is required to prevent epithelial fusions characteristic for popliteal pterygium syndromes.Cell Death Differ.22, 1012–1024 (2015).
Kalay, E. et al. Mutations inRIPK4cause the autosomal-recessive form of popliteal pterygium syndrome.Am. J. Hum. Genet.90, 76–85 (2012).
Massoud, A. A., Ammaari, A. N., Khan, A. S. S., ven Katraman, B. & Teebi, A. S. Bartsocas–Papas syndrome in an Arab family with four affected sibs: further characterization.Am. J. Med. Genet.79, 16–21 (1998).
Van Der Woude, A. Fistula labii inferioris congenita and its association with cleft lip and palate.Am. J. Hum. Genet.6, 244–256 (1954).
Butali, A. et al. NovelIRF6mutations in families with Van Der Woude syndrome and popliteal pterygium syndrome from sub-Saharan Africa.Mol. Genet. Genomic Med.2, 254–260 (2014).
Gorlin, R. J., Sedano, H. O. & Cervenka, J. Popliteal pterygium syndrome. a syndrome comprising cleft lip-palate, popliteal and intercrural pterygia, digital and genital anomalies.Pediatrics41, 503–509 (1968).
Richardson, R. J. et al. Irf6 is a key determinant of the keratinocyte proliferation-differentiation switch.Nat. Genet.38, 1329–1334 (2006).
Kwa, M. Q. et al. Receptor-interacting protein kinase 4 and interferon regulatory factor 6 function as a signaling axis to regulate keratinocyte differentiation.J. Biol. Chem.289, 31077–31087 (2014).
Huang, C. S. et al. Crystal structure of Ripk4 reveals dimerization-dependent kinase activity.Structure26, 767–777 (2018).
Dassule, H. R., Lewis, P., Bei, M., Maas, R. & McMahon, A. P. Sonic hedgehog regulates growth and morphogenesis of the tooth.Development127, 4775–4785 (2000).
Kousa, Y. A., Moussa, D. & Schutte, B. C. IRF6 expression in basal epithelium partially rescuesIrf6knockout mice.Dev. Dyn.246, 670–681 (2017).
Urwyler-Rösselet, C. et al. Keratinocyte-specific ablation of RIPK4 allows epidermal cornification but impairs skin barrier formation.J. Invest. Dermatol.138, 1268–1278 (2018).
Chen, W. et al. Insights into interferon regulatory factor activation from the crystal structure of dimeric IRF5.Nat. Struct. Mol. Biol.15, 1213–1220 (2008).
Matsuzawa, N. et al. Two missense mutations of theIRF6gene in two Japanese families with popliteal pterygium syndrome.Am. J. Med. Genet.152A, 2262–2267 (2010).
Botti, E. et al. Developmental factor IRF6 exhibits tumor suppressor activity in squamous cell carcinomas.Proc. Natl Acad. Sci. USA108, 13710–13715 (2011).
Liu, H. et al. Irf6 directly regulates Klf17 in zebrafish periderm and Klf4 in sign oral epithelium, and dominant-negative KLF4 variants are present in patients with cleft lip and palate.Hum. Mol. Genet.25, 766–776 (2016).
Little, H. J. et al. Missense mutations that cause Van der Woude syndrome and popliteal pterygium syndrome affect the DNA-binding and transcriptional activation functions of IRF6.Hum. Mol. Genet.18, 535–545 (2009).
Voigt, P., Tee, W. W. & Reinberg, D. A double take on bivalent promoters.Genes Dev.27, 1318–1338 (2013).
de la Garza, G. et al. Interferon regulatory factor 6 promotes differentiation of the periderm by activating expression of Grainyhead-like 3.J. Invest. Dermatol.133, 68–77 (2013).
Peyrard-Janvid, M. et al. Dominant mutations inGRHL3cause Van der Woude syndrome and disrupt oral periderm development.Am. J. Hum. Genet.94, 23–32 (2014).
Ting, S. B. et al. A homolog ofDrosophilagrainy head is essential for epidermal integrity in mice.Science308, 411–413 (2005).
Furuse, M. et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice.J. Cell Biol.156, 1099–1111 (2002).
Li, S. et al. Transcription factor CTIP1/ BCL11A regulates epidermal differentiation and lipid metabolism during skin development.Sci. Rep.7, 13427 (2017).
Dai, J., Brooks, Y., Lefort, K., Getsios, S. & Dotto, G. P. The retinoid-related orphan receptor RORα promotes keratinocyte differentiation via FOXN1.PLoS ONE8, e70392 (2013).
Grond, S. et al. PNPLA1 deficiency in mice and humans leads to a defect in the synthesis of omega-O-acylceramides.J. Invest. Dermatol.137, 394–402 (2017).
Eckl, K. M. et al. Impaired epidermal ceramide synthesis causes autosomal recessive congenital ichthyosis and reveals the importance of ceramide acyl chain length.J. Invest. Dermatol.133, 2202–2211 (2013).
Jennemann, R. et al. Loss of ceramide synthase 3 causes lethal skin barrier disruption.Hum. Mol. Genet.21, 586–608 (2012).
Ryder, E. et al. Rapid conversion of EUCOMM/KOMP-CSD alleles in mouse embryos using a cell-permeable Cre recombinase.Transgenic Res.23, 177–185 (2014).
Hafner, M. et al. Keratin 14 Cre transgenic mice authenticate keratin 14 as an oocyte-expressed protein.Genesis38, 176–181 (2004).
DiTommaso, T. & Foijer, F. Barrier function assay.Bio Protoc.4, e1133 (2014).
Gareus, R. et al. Normal epidermal differentiation but impaired skin-barrier formation upon keratinocyte-restricted IKK1 ablation.Nat. Cell Biol.9, 461–469 (2007).
Hanley, K. et al. Glucocorticoid deficiency delays stratum corneum maturation in the fetal mouse.J. Invest. Dermatol.111, 440–444 (1998).
Smyth, G. K. inBioinformatics and Computational Biology Solutions Using R and Bioconductor(eds Gentleman, R., Carey, V. J., Huber, W., Irizarry, R. A. & Dudoit, S.) 397–420 (Springer, New York, 2005).
R Core Team.R: A language and environment for statistical computing.(R Foundation for Statistical Computing, 2018).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies.Nucleic Acids Res.43, e47 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2.Genome Biol.15, 550 (2014).
Alhamdoosh, M. et al. Combining multiple tools outperforms individual methods in gene set enrichment analyses.Bioinformatics33, 414–424 (2017).
Zhang, Y. et al. Model-based analysis of ChIP–seq (MACS).Genome Biol.9, R137 (2008).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors primecis-regulatory elements required for macrophage and B cell identities.Mol. Cell38, 576–589 (2010).
Hahne, F. & Ivanek, R. Visualizing genomic data using Gviz and bioconductor.Methods Mol. Biol.1418, 335–351 (2016).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data.Bioinformatics32, 2847–2849 (2016).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position.Nat. Methods10, 1213–1218 (2013).
Corces, M. R. et al. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues.Nat. Methods14, 959–962 (2017).
Bailey, T. L. et al. MEME SUITE: tools for motif discovery and searching.Nucleic Acids Res.37, W202–W208 (2009).
Beausoleil, S. A., Villén, J., Gerber, S. A., Rush, J. & Gygi, S. P. A probability-based approach for high-throughput protein phosphorylation analysis and site localization.Nat. Biotechnol.24, 1285–1292 (2006).
Elias, J. E. & Gygi, S. P. Target-decoy search strategy for mass spectrometry-based proteomics.Methods Mol. Biol.604, 55–71 (2010).
Acknowledgements
We thank T. Scholl, B. Halpenny, K. Veliz, C. Dela Cruz, L. Orellana and F. Gallardo for animal husbandry; and M. Chen, A. Pierce and the Genentech genetic analysis and histology laboratories for technical assistance.
Author information
Authors and Affiliations
Contributions
M.R.-G. and S.W. generated theRipk4+/loxp,Irf6+/loxp,Irf6+/S413A,S424AandIrf6+/S413E,S424Emice; N.O. designed and performed experiments with help from A.M. and D.L.D; R.R. and Y.Z. performed bioinformatic analysis; C.S.H. purified recombinant proteins; V.C.P. performed mass spectrometry with help from K.Y., Q.L., A.B. and W.W; W.S. performed lipidomics; L.G.K. performed confocal microscopy; J.D.W. analysed histological data; and V.M.D. and K.N. helped with experimental design.
Corresponding author
Ethics declarations
Competing interests
All authors are employees of Genentech.
Additional information
Publisher’s noteSpringer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peer review informationNaturethanks Salvador Aznar Benitah, Ya-Chieh Hsu, Etienne Meylan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
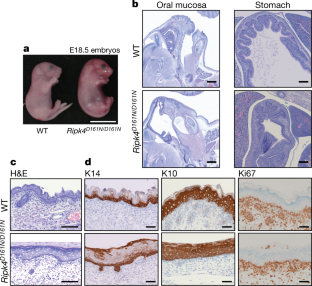
Extended Data Fig. 1Ripk4D161N/D161Nmice are neonatal lethal.
a, Organization of the constitutiveRipk4D161Nknock-in allele. Boxes represent exons. Untranslated regions are shaded grey. Not to scale.b, Western blots of wild-type,Ripk4+/D161NandRipk4D161N/D161NE16.5 skin. Representative of three independent experiments.c, Table showing the observed and expected numbers at clipping (P4–P7) of offspring that were generated from intercrossingRipk4+/D161Nmice.d–g, Organization of the conditionalRipk4knockout allele (d), conditionalIrf6knockout allele (e), constitutiveIrf6S413A,S424Aknock-in allele (f) and constitutiveIrf6S413E,S424Eknock-in allele (g). Boxes represent exons. Untranslated regions are shaded grey. Not to scale.
Extended Data Fig. 2 Keratinocyte-specific deletion ofIrf6orRipk4demonstrates their cell-autonomous requirement for epidermal differentiation.
a, Western blots of epidermis and fetal liver from an E18.5Irf6EKOembryo (K14-Cre+;Irf6loxp/loxp) and its K14-Cre+;Irf6+/loxp同窝出生仔畜控制。正如所料,有问题t deletion of IRF6 in the epidermis only. Representative of three independent experiments.b, Table showing the observed and expected numbers at clipping (P4–P7) of offspring that were generated from intercrossingIrf6loxp/loxp; K14-Cre−females andIrf6+/loxp; K14-Cre+males. NoIrf6EKOmice were observed.c, P0Irf6EKOand its littermate control (n= 3 each). Scale bar, 1 cm.d, Toluidine blue staining of P0Irf6EKOand control embryos (n= 3 each). Scale bar, 1 cm.e, E18.5 skin sections from control andIrf6EKOembryos (n= 3 each) stained with H&E or antibodies against K10, K14 and Ki67. Scale bars, 50 μm.f, E18.5 sections from control andIrf6EKOembryos (n= 3 each) stained with H&E, showing no fusion of the squamous epithelium at the mouth. Scale bars, 200 μm.g, Western blots of epidermis and whole skin from an E18.5Ripk4EKOembryo (K14-Cre+;Ripk4loxp/loxp) and its K14-Cre+;Ripk4+/loxp同窝出生仔畜控制。正如所料,有问题t deletion ofRipk4in the epidermis only. Representative of three independent experiments.h, Table showing the observed and expected numbers at clipping (P4–P7) of offspring that were generated from intercrossingRipk4loxp/loxp; K14-Cre−females andRipk4+/loxp; K14-Cre+males. NoRipk4EKOmice were observed.i, P0Ripk4EKOand its littermate control (n= 3 each). Scale bar, 1 cm.j, Toluidine blue staining of P0Ripk4EKOand control embryos (n= 3 each). Scale bar, 1 cm.k, E18.5 skin sections from control andRipk4EKOembryos, stained with H&E or immunolabelled with antibodies against K10, K14 and Ki67. Scale bars, 50 μm.l, E18.5 sections from control andRipk4EKOembryos stained with H&E, showing no fusion of the squamous epithelium at the mouth and no fusion of the tongue to the palate. Scale bars, 800 μm.
Extended Data Fig. 3 The epidermis of E18.5Ripk4D161N/D161NIrf6−/−embryos is indistinguishable from that ofIrf6−/−embryos.
a, E18.5 sections with the indicated genotypes (n= 3 each) stained with H&E, showing fusion of the oral mucosa (arrows), irregular incisors with premature eruption (asterisks) and fusion of the stratified squamous portion of the stomach in theIrf6−/−andRipk4D161N/D161NIrf6−/−embryos. Scale bars, 200 μm (oral mucosa); 100 μm (stomach).b, E18.5 skin sections with the indicated genotypes (n= 3) immunolabelled with antibodies against K10, K14 and Ki67. Scale bars, 50 μm.c, Table showing the observed and expected numbers at clipping (P4–P7) of offspring that were generated from intercrossingIrf6+/−Ripk4+/D161Nand wild-type mice.Irf6+/−Ripk4+/D161Noffspring were weaned at the expected numbers.
Extended Data Fig. 4 Phosphorylation of IRF6 at Ser413 and S424 in vivo is essential for epidermal differentiation and development.
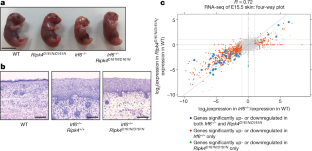
a, 3×FLAG-tagged IRF6 was affinity-purified from 293T cells co-expressing Myc-tagged RIPK4 or RIPK4(D161N) and then stained with SimplyBlue (left) or western blotted (right). IP, immunoprecipitation. Results representative of two independent experiments.b, Extracted ion chromatograms (XIC) of the phosphorylated peptides and their corresponding unmodified counterparts at pS424 (LQISTPDIKDNIVAQLK) (i) and pS(413,416) (SFDSGSVR)(ii); both the endogenous (light label) and the synthetic (heavy label) isotopically labelled peptides are shown. Plots are representative of two independent experiments.c, Absolute quantification at the pS413 and pS416 sites is difficult because peptides covering these sites co-elute. An experiment was therefore performed using IRF6(S413A) and IRF6(S416A) to distinguish between the levels of phosphorylation at these two sites. The resulting extracted ion chromatograms of the peptides without heavy labelling are shown, with the corresponding (AUC) values in the table below (RT, retention time). On the basis of the AUCs, there is more pS413 in the IRF6(S416A) sample than pS416 in the IRF6(S413A) sample. Given that the level of the unmodified form is similar, this suggests that S413 is the main phosphorylation site. This conclusion assumes that the ionization efficiency of these two peptides is the same, and is therefore semi-quantitative.d, Left, graph indicates activation of an IRF-responsive luciferase reporter gene at 24 h after transfection of 293T cells with the indicated IRF6 and RIPK4 constructs. IRF6 activity is displayed as fold activity over reporter only. Data are mean ± s.d (n= 6). Unpaired, two-tailed Fisher’s exact test with 95% confidence interval. Right, western blots show expression of the IRF6 phosphorylation mutants that were used in the luciferase assay. Representative of three independent experiments.e, Production of knock-in mutant mice expressing IRF6(S413A/S424A). Representative genomic sequencing of wild-type and homozygous (Irf6S413A,S424A/ S413A,S424A) mice. Nucleotides that encode Ser413 and Ser424 are highlighted by the dashed boxes, which indicate the wild-type GCC (Ser) and homozygous knock-in mutation TCC (Ala).f, Genome-wide four-way plot showing genes that have increased or decreased expression inIrf6S413A,S424A/S413A,S424A(yaxis,n= 5) orIrf6−/−(xaxis,n= 5)比野生型(n= 3) in E15.5 skin. Each coloured dot represents a gene that met the cut-offs of an adjustedPvalue <0.05 and a minimum twofold change in expression. AdjustedPvalues were obtained using a moderatedt-test (two-sided) with the Benjamini–Hochberg method for multiple comparisons. Genes that were altered significantly in expression inIrf6−/−skin only are shown in red, those altered inIrf6S413A,S424A/S413A,S424Aonly are shown in green and those altered in both genotypes are shown in blue. The Pearson correlation coefficient (Rvalue) is 0.72.
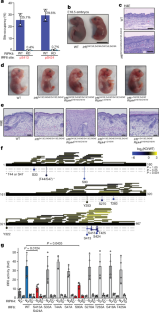
Extended Data Fig. 5 The IRF6 phosphomimetic knock-in mouse demonstrates that phosphorylation of IRF6 at Ser413 and Ser424 is essential to prime it for activation.
a, Western blots of wild-type,Irf6+/S413A,S424AandIrf6S413A,S424A/S413A,S424AE18.5 skin. Representative of three independent experiments.b, E18.5 skin sections (n= 3 wild type andn= 3Irf6S413A,S424A/S413A,S424A) stained with H&E or antibodies against K10, K14 and Ki67. Scale bars, 50 μm.c, E18.5 sections (n= 3 wild type andn= 3Irf6S413A,S424A/S413A,S424A) stained with H&E, showing fusion of the stratified squamous portion of the stomach in theIrf6S413A,S424A/S413A.S424Aembryos. Scale bars, 100 μm.d, Graph indicates activation of an IRF-responsive luciferase reporter gene at 24 h after transfection of 293T cells with the indicated IRF6 and RIPK4 constructs. IRF6 activity is displayed as fold activity over reporter only. Data are mean ± s.d. (n= 5). IRF6(R84C) is a DNA-binding mutant, and represents a negative control in this experiment. Unpaired two-tailed Fisher’s exact test with 95% confidence interval.e, Western blots of wild-type,Irf6S413E,S424E/S413E,S424EandIrf6−/−E18.5 skin. Representative of three independent experiments.f, E18.5 skin sections from wild-type,Irf6S413E,S424E/S413E,S424E,Irf6S413E,S424E/S413E,S424ERipk4D161N/D161N,Irf6S413E,S424E/S413E,S424ERipk4+/D161NandIrf6+/S413E,S424ERipk4D161N/D161Nembryos (n= 3 each) stained with antibodies against K10, K14 and Ki67. Scale bars, 50 μm.g, E18.5 sections from wild-type,Irf6S413E,S424E/S413E,S424E,Irf6S413E,S424E/S413E,S424ERipk4D161N/D161N,Irf6S413E,S424E/S413E,S424ERipk4+/D161NandIrf6+/S413E,S424ERipk4D161N/D161Nembryos (n= 3 each) stained with H&E, showing fusion of the squamous epithelium at the mouth and fusion of the tongue to the palate. Scale bars, 100 μm.h, Table showing the observed and expected numbers at E18.5 of offspring that were generated from intercrossingRipk4+/D161NIrf6+/S413E,S424Emice.i, Table showing the observed and expected numbers at E18.5 of offspring that were generated from intercrossingIrf6+/S413E.S424Emice.
Extended Data Fig. 6 SILAC identifies Ser90 as an additional IRF6 phosphorylation site that is essential for its activation in vitro.
a, Schematic of the SILAC experiment that was performed to identify additional RIPK4-dependent phosphorylation sites on IRF6.b, Western blots of 293T cells expressing the IRF6 phosphorylation mutants that were used in luciferase reporter assays. Representative of three independent experiments.c, Schematic of the humanIRF6locus. Exons are shown as rectangles, introns as interconnecting lines and untranslated regions are shaded in grey. The DNA-binding domain (DNA BD), IRF-association domain (IRF AD) and C-terminal domain (CTD) are highlighted in blue, green and orange, respectively. The relative positions of two patient mutations, S90G (which gives rise to VWS) and S424L (which gives rise to PPS) are displayed in red. The locus is drawn approximately to scale.d, Extracted ion chromatograms of phosphorylated peptides and their unmodified counterparts at pS(413,416) (SFDSGSVR) (i), pS424 (short peptide: LQISTPDIK) (ii), pS424 (long peptide: LQISTPDIKDNIVAQLK)(iii)and pS90 (SREFNLMoxYDGTK)(iv).Recombinant full-length IRF6 was incubated with the RIPK4 kinase domain (either wild type or T184I (a BPS mutation that produces a kinase-dead version of RIPK4) for 5 and 30 min. No phosphorylation was observed when IRF6 was incubated with RIPK4(T184I). Representative of two experiments.e, Proposed model for IRF6 regulation by RIPK4. RIPK4 (or kinase(s) X) phosphorylates IRF6 at Ser413 and Ser424, which act as ‘priming’ sites. Priming enhances the phosphorylation of IRF6 by RIPK4 at an additional site that is essential for IRF6 activation, Ser90. This allows normal skin differentiation and development. In the first scenario, in whichIrf6is mutated toIrf6S413,S424A/S413A,S424A, RIPK4 (or other kinases) cannot phosphorylate Ser413 and Ser424. IRF6 is thus non-functional, so theIrf6S413A,S424A/S413A,S424A敲入小鼠ph值enocopies theIrf6−/−mouse. In the second scenario, in whichIrf6is mutated toIrf6S413E,S424E/S413E,S424E, the Glu residues at Ser413 and Ser424 mimic priming and allow RIPK4 to phosphorylate IRF6 at Ser90. Thus, IRF6 is functional, andIrf6S413E,S424Eresembles the wild type. In the third, double-mutant scenario (Irf6S413E,S424E/S413E,S424ERipk4D161N/D161N), despite effective IRF6 priming at Ser413 and Ser424 as a result of Glu substitutions, RIPK4 is kinase-dead and therefore cannot phosphorylate IRF6 at Ser90 and activate it. Thus, IRF6 is non-functional and this double mutant phenocopies theIrf6−/−mouse. In the final scenario (Irf6+/S413E.S424ERipk4D161N/D161N), one wild-type allele ofIrf6is present (sufficient for normal IRF6 function); however, there is no RIPK4 kinase activity. Therefore, these mice phenocopyRipk4D161N/D161Nmice.
Extended Data Fig. 7 SILAC experiments demonstrate that mutating IRF6 Ser413 and Ser424 to Ala reduces the amount of phosphorylation at IRF6 Ser90 by half.
a, Schematic of the SILAC experiment that was performed to compare the amount of phosphorylation at S90, S413 and S424 between wild-type and mutant IRF6.b, Extracted ion chromatograms of the peptide phosphorylated at Ser90 (pSREFNLMoxYDGTK) and its unmodified counterpart (SREFNLMoxYDGTK), from wild-type IRF6 (light labelled) and IRF6(S413A/S424A) (heavy labelled). More phosphorylation at Ser90 was observed in wild-type IRF6 than in IRF6(S413A/S424A) (log2(S413A.S424A/WT) = −0.5) even though there was more total IRF6(S413A/S424A) (log2(S413A.S424A/WT) = 0.6). When normalized for total IRF6 levels, log2(S413A.S424A / WT)≈−1.1;也就是说,2.1倍少pS90 in IRF6(S413A/S424A) compared to wild-type IRF6.c, Extracted ion chromatograms of the peptide phosphorylated at Ser413 or Ser416 (pSFDSGSVR) and its unmodified counterpart (SFDSGSVR), from wild-type IRF6 (light labelled) and IRF6(S90A) (heavy labelled). When normalized for total IRF6 levels, log2(S90A / WT) = 0.11,所以pS413 similar level in wild-type IRF6 and IRF6(S90A).d, Extracted ion chromatograms of the peptide phosphorylated at Ser424 (long peptide: LQIpSTPDIKDNIVAQLK) and its unmodified counterpart (long peptide: LQISTPDIKDNIVAQLK), from wild-type IRF6 (light labelled) and IRF6(S90A) (heavy labelled). pS424 is at a similar level in wild-type IRF6 and IRF6(S90A) (log2(S90A/WT) = 0.2).e, Extracted ion chromatograms of the peptide phosphorylated at Ser90 (pSREFNLMoxYDGTK) and its unmodified counterpart (SREFNLMoxYDGTK), from IRF6(S423A/S424A) (light labelled) and IRF6(S413E/S424E) (heavy labelled). More pS90 was observed in IRF6(S413E/S424E) than in IRF6(S413A/S424A) (log2(S413E.S424E/S413A.S424A) = 0.6) even though there was slightly less total IRF6(S413E/S424E) (log2(S413E.S424E/S413A.S424A) = −0.4). When normalized for total IRF6 levels, log2(S413E.S424E/S413A.S424A) ≈ 1.0; that is, twofold less pS90 in IRF6(S413A/S424A) compared to IRF6(S413E/S424E).f, Extracted ion chromatograms of the peptide phosphorylated at Ser90 (pSREFNLMoxYDGTK) and its unmodified counterpart (SREFNLMoxYDGTK), from wild-type IRF6 (light labelled) and IRF6(S413E/S424E) (heavy labelled). More pS90 was observed in IRF6(S413E/S424E) than in wild-type IRF6 (log2(S413E.S424E/WT) = 0.7) even though there was also slightly more total IRF6(S413E/S424E) (log2(S413E.S424E/WT) = 0.1). When normalized for total IRF6 levels, log2(S413E.S424E/WT) ≈ 0.6; that is, 1.52-fold more pS90 in IRF6(S413E/S424E) compared to wild-type IRF6. Chromatograms are representative of two experiments. Notably, these are extracted ion chromatograms of representative peptide spectral matches (PSMs). The reported log2values were calculated from all the different types of PSMs that cover the phosphorylation sites of interest.
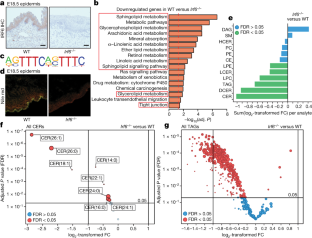
Extended Data Fig. 8 Bioinformatic analysis showing that IRF6 target genes are involved in lipid metabolism and tight-junction formation, which are essential for epidermal barrier function.
a,分析管道使用的示意图to identify putative and high-confidence IRF6 targets. The histone ChIP–seq dataset that yielded an IRF6 motif (the ISRE) is marked in red.b, Heat map showing clustering of 35,176 genes into four clusters: active, repressed, bivalent and low-signal promoter groups (top to bottom) (n= 11,942,n= 2,580,n= 3,154 andn= 17,500, respectively). Each row reports log2-transformed reads per million (RPM) in a 5,000-bp window around the start of the gene for H3K4me3 (left two columns) and H3K27me3 (right two columns) in the wild-type background.c, Distribution of log2-transformed read counts in a 5,000-bp window (binned at 50 bp) around 815 putative IRF6 targets (54 out of 869 targets were filtered out owing to low signal) for H3K4me3, H3K27me3, H3K27ac and ATAC-seq signal in the wild-type background, sorted in the same way asb.The number of putative IRF6 targets in active, repressed, bivalent and low-signal groups are 412, 64, 129 and 210, respectively. Genes that are significantly up- or downregulated inIrf6−/−versus wild-type E16.5 skin (n= 3 each, from the RNA-seq dataset) are shown in red and blue to the right.Pvalues were obtained using a moderatedt-test (two-sided) with the Benjamini–Hochberg method for multiple comparisons.d, Toluidine blue staining of wild-type andIrf6−/−E18.5 embryos (n= 3 each). Scale bar, 1 cm.e, Graph showing weight loss of wild-type control (n= 4) andIrf6−/−(n= 4) newborn mice over time. Data are mean ± s.d. Unpaired, two-tailed Fisher’s exact test with 95% confidence interval.f–h, Screenshots showing normalized read coverage for ATAC-seq, H3K4me3, H3K27ac and H3K27me3 in wild-type andIrf6−/−skin for occludin (Ocln) (e),Pnpla1(f) andGrhl3(g). Genes are indicated in blue in the top panel; red boxes highlight the signal at the start of the gene. Representative ofn= 2 for wild type andIrf6−/−.
Extended Data Fig. 9 Lipid profiling showing a decrease in the quantity of certain classes of lipids inIrf6−/−compared to wild-type skin.
a, Graphs of relative mRNA expression ofGrhl3,Ocln,Pnpla1,Sptlc3,Cers3andRorain E15.5 skin. Each circle represents a different embryo,n= 3. Centre represents the mean; error bars denote s.d. Unpaired, two-tailed Fisher’s exact test with 95% confidence interval.b, Quantification of 12 classes of lipids in wild-type andIrf6−/−E16.5 skin (n= 4 each) (nmol per g of wet skin). Data are mean ± s.d. FDRs (or adjustedPvalue) of <0.05 are shown on the graph.Pvalues were obtained using a moderatedt-test (two-sided) with the Benjamini–Hochberg method for multiple comparisons.c, Quantification of four different species of HCERs in wild-type andIrf6−/−E16.5 skin (n= 4 each) (nmol per g of wet skin). Each dot represents one skin sample. Centre represents the mean; error bars denote s.d. FDRs of <0.05 are labelled on the graph.Pvalues were obtained using a moderatedt-test (two-sided) with the Benjamini–Hochberg method for multiple comparisons. There is a significant decrease in species with ultra-long-chain acyl moieties (≥C26) inIrf6−/−compared to wild-type skin.d, Quantification of four different species of DCERs in wild-type andIrf6−/−E16.5 skin (n= 4 each) (nmol per g of wet skin). Each dot represents one skin sample. Centre represents the mean; error bars denote s.d. FDRs of <0.05 are labelled on the graph.Pvalues were obtained using a moderatedt-test (two-sided) with the Benjamini–Hochberg method for multiple comparisons. There is a significant decrease in species with ultra-long-chain acyl moieties (≥C26) inIrf6−/−compared to wild-type skin.
Extended Data Fig. 10 Lipid profiling showing a decrease in the quantity of certain classes of lipids inRipk4D161N/D161Ncompared to wild-type skin.
a, E18.5 skin sections from wild-type andRipk4D161N/D161Nembryos (n= 3) were stained with Nile red fluorescent dye, which indicates polar lipids in red and non-polar lipids in green. 72.66 × 72.66 microns.b, Profiling of 12 classes of lipids in wild-type andRipk4D161N/D161NE16.5 skin. Thexaxis denotes the sum(log2(abundance inRipk4D161N/D161N/abundance in wild type)) per analyte; values less than 0 indicate a decrease, and values greater than 0 an increase, inRipk4D161N/D161Nversus wild-type skin. Green bars denote a significant FDR of <0.05. Moderatedt-test (two-sided).Pvalues were obtained using a moderatedt-test (two-sided) with the Benjamini–Hochberg method for multiple comparisons. There are significantly lower levels of CERs, DCERs and LCERs inRipk4D161N/D161Ncompared to wild-type skin. Levels of the other classes of lipids are unchanged.c, Quantification of 12 classes of lipids in wild-type andRipk4D161N/D161NE16.5 skin (n= 4) (nmol per g of wet skin). Each dot represents one skin sample. Centre represents the mean; error bars denote s.d. FDRs of <0.05 are shown on the graph.Pvalues were obtained using a moderatedt-test (two-sided) with the Benjamini–Hochberg method for multiple comparisons.
Supplementary information
Supplementary Figure 1
Uncropped blots for Figures 2 and 3 and Extended Data Figures 1, 3, 4 and 6.
Supplementary Table
Sequences of the AQUA peptides used for the proteomics experiment in Figure 3a and Extended Data Figure 4a-c.
Rights and permissions
About this article
Cite this article
Oberbeck, N., Pham, V.C., Webster, J.D.et al.The RIPK4–IRF6 signalling axis safeguards epidermal differentiation and barrier function.Nature574, 249–253 (2019). https://doi.org/10.1038/s41586-019-1615-3
Received:
Accepted:
Published:
Issue Date:
DOI:https://doi.org/10.1038/s41586-019-1615-3
This article is cited by
Genome-wide meta-analysis of monoclonal gammopathy of undetermined significance (MGUS) identifies risk loci impacting IRF-6
Blood Cancer Journal(2022)
Inhibition of KIF20A by transcription factor IRF6 affects the progression of renal clear cell carcinoma
Cancer Cell International(2021)
Loss of FOXC1 contributes to the corneal epithelial fate switch and pathogenesis
Signal Transduction and Targeted Therapy(2021)
Spatial competition shapes the dynamic mutational landscape of normal esophageal epithelium
Nature Genetics(2020)
Comments
By submitting a comment you agree to abide by ourTermsandCommunity Guidelines.If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
