Abstract
Increasing soil carbon and nitrogen storage can help mitigate climate change and sustain soil fertility1,2.A large number of biodiversity-manipulation experiments collectively suggest that high plant diversity increases soil carbon and nitrogen stocks3,4.It remains debated, however, whether such conclusions hold in natural ecosystems5,6,7,8,9,10,11,12.这里我们分析加拿大国家森林Inventory (NFI) database with the help of structural equation modelling (SEM) to explore the relationship between tree diversity and soil carbon and nitrogen accumulation in natural forests. We find that greater tree diversity is associated with higher soil carbon and nitrogen accumulation, validating inferences from biodiversity-manipulation experiments. Specifically, on a decadal scale, increasing species evenness from its minimum to maximum value increases soil carbon and nitrogen in the organic horizon by 30% and 42%, whereas increasing functional diversity enhances soil carbon and nitrogen in the mineral horizon by 32% and 50%, respectively. Our results highlight that conserving and promoting functionally diverse forests could promote soil carbon and nitrogen storage, enhancing both carbon sink capacity and soil nitrogen fertility.
This is a preview of subscription content,access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 per month
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Get just this article for as long as you need it
$39.95
Prices may be subject to local taxes which are calculated during checkout
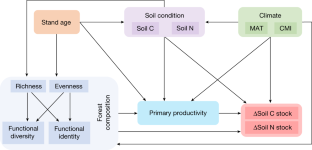
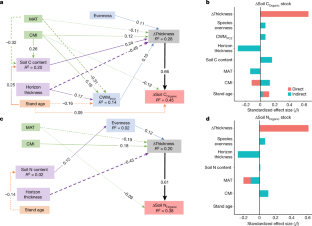
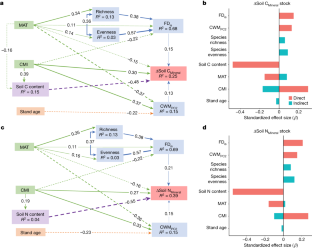
Data availability
The source data underlying Figs.2and3are provided as source data files and all data used in this study are archived in Figshare (https://doi.org/10.6084/m9.figshare.20988187.v2).Source dataare provided with this paper.
Code availability
The R scripts needed to reproduce the analysis are archived in Figshare (https://doi.org/10.6084/m9.figshare.20988187.v2).
References
Lal, R. Soil carbon sequestration impacts on global climate change and food security.Science304, 1623–1627 (2004).
Vitousek, P. M. & Howarth, R. W. Nitrogen limitation on land and in the sea: how can it occur?.Biogeochemistry13, 87–115 (1991).
Chen, X. L., Chen, H. Y. H., Searle, E. B., Chen, C. & Reich, P. B. Negative to positive shifts in diversity effects on soil nitrogen over time.Nat. Sustain.4, 225–234 (2021).
Chen, X. et al. Effects of plant diversity on soil carbon in diverse ecosystems: a global meta-analysis.Biol. Rev.95, 167–183 (2020).
Chen, S. P. et al. Plant diversity enhances productivity and soil carbon storage.Proc. Natl Acad. Sci. USA115, 4027–4032 (2018).
Chen, X., Hisano, M., Taylor, A. R. & Chen, H. Y. H. The effects of functional diversity and identity (acquisitive versus conservative strategies) on soil carbon stocks are dependent on environmental contexts.For. Ecol. Manag.503, 119820 (2022).
Dawud, S. M. et al. Is tree species diversity or species identity the more important driver of soil carbon stocks, C/N ratio, and pH?Ecosystems19, 645–660 (2016).
Conti, G. & Diaz, S. Plant functional diversity and carbon storage – an empirical test in semi-arid forest ecosystems.J. Ecol.101, 18–28 (2013).
van der Plas, F. Biodiversity and ecosystem functioning in naturally assembled communities.Biol. Rev.94, 1220–1245 (2019).
Gamfeldt, L. et al. Higher levels of multiple ecosystem services are found in forests with more tree species.Nat. Commun.4, 1340 (2013).
Dawud, S. M. et al. Tree species functional group is a more important driver of soil properties than tree species diversity across major European forest types.Funct. Ecol.31, 1153–1162 (2017).
Ratcliffe, S. et al. Biodiversity and ecosystem functioning relations in European forests depend on environmental context.Ecol. Lett.20, 1414–1426 (2017).
Carvalhais, N. et al. Global covariation of carbon turnover times with climate in terrestrial ecosystems.Nature514, 213–217 (2014).
Lange, M. et al. Plant diversity increases soil microbial activity and soil carbon storage.Nat. Commun.6, 6707 (2015).
Mao, Z. K. et al. Tree mycorrhizal associations mediate soil fertility effects on forest community structure in a temperate forest.New Phytol.223, 475–486 (2019).
Zhang, Y., Chen, H. Y. H. & Taylor, A. R. Positive species diversity and above-ground biomass relationships are ubiquitous across forest strata despite interference from overstorey trees.Funct. Ecol.31, 419–426 (2017).
Post, W. M., Pastor, J., Zinke, P. J. & Stangenberger, A. G. Global patterns of soil nitrogen storage.Nature317, 613–616 (1985).
京,x et al。以上,地下补arity rather than selection drive tree diversity–productivity relationships in European forests.Funct. Ecol.35, 1756–1767 (2021).
张超,T Bouriaud, O Avacaritei, d &锯屑,D. A. Stabilizing effects of diversity on aboveground wood production in forest ecosystems: linking patterns and processes.Ecol. Lett.17, 1560–1569 (2014).
Jucker, T. et al. Climate modulates the effects of tree diversity on forest productivity.J. Ecol.104, 388–398 (2016).
van der Voort, T. S. et al. Variability in14C contents of soil organic matter at the plot and regional scale across climatic and geologic gradients.Biogeosciences13, 3427–3439 (2016).
Grace, J. B. et al. Integrative modelling reveals mechanisms linking productivity and plant species richness.Nature529, 390–393 (2016).
Shovon, T. A., Kang, S., Scherer-Lorenzen, M. & Nock, C. A. Changes in the direction of the diversity–productivity relationship over 15 years of stand development in a planted temperate forest.J. Ecol.110, 1125–1137 (2022).
De Deyn, G. B., Cornelissen, J. H. & Bardgett, R. D. Plant functional traits and soil carbon sequestration in contrasting biomes.Ecol. Lett.11, 516–531 (2008).
Paquette, A. & Messier, C. The effect of biodiversity on tree productivity: from temperate to boreal forests.Glob. Ecol. Biogeogr.20, 170–180 (2011).
Weedon, J. T. et al. Global meta-analysis of wood decomposition rates: a role for trait variation among tree species?Ecol. Lett.12, 45–56 (2009).
Chave, J. et al. Towards a worldwide wood economics spectrum.Ecol. Lett.12, 351–366 (2009).
Adair, E. C., Hooper, D. U., Paquette, A. & Hungate, B. A. Ecosystem context illuminates conflicting roles of plant diversity in carbon storage.Ecol. Lett.21, 1604–1619 (2018).
Hillebrand, H., Bennett, D. M. & Cadotte, M. W. Consequences of dominance: a review of evenness effects on local and regional ecosystem processes.Ecology89, 1510–1520 (2008).
Jackson, R. B. et al. The ecology of soil carbon: pools, vulnerabilities, and biotic and abiotic controls.Annu. Rev. Ecol. Evol. Syst.48, 419–445 (2017).
Chen, X. & Chen, H. Y. H. Global effects of plant litter alterations on soil CO2to the atmosphere.Glob. Change Biol.24, 3462–3471 (2018).
Pietsch, K. A. et al. Global relationship of wood and leaf litter decomposability: the role of functional traits within and across plant organs.Glob. Ecol. Biogeogr.23, 1046–1057 (2014).
Rosell, J. A., Gleason, S., Mendez-Alonzo, R., Chang, Y. & Westoby, M. Bark functional ecology: evidence for tradeoffs, functional coordination, and environment producing bark diversity.New Phytol.201, 486–497 (2014).
Kahl, T. et al. Wood decay rates of 13 temperate tree species in relation to wood properties, enzyme activities and organismic diversities.For. Ecol. Manag.391, 86–95 (2017).
Giardina, F. et al. Tall Amazonian forests are less sensitive to precipitation variability.Nat. Geosci.11, 405–409 (2018).
Park, J. H. & Matzner, E. Controls on the release of dissolved organic carbon and nitrogen from a deciduous forest floor investigated by manipulations of aboveground litter inputs and water flux.Biogeochemistry66, 265–286 (2003).
Olson, J. S. Energy storage and the balance of producers and decomposers in ecological systems.Ecology44, 322–331 (1963).
Hassink, J. The capacity of soils to preserve organic C and N by their association with clay and silt particles.Plant Soil191, 77–87 (1997).
Clemmensen, K. E. et al. Roots and associated fungi drive long-term carbon sequestration in boreal forest.Science339, 1615–1618 (2013).
Preston, C. M., Bhatti, J. S., Flanagan, L. B. & Norris, C. Stocks, chemistry, and sensitivity to climate change of dead organic matter along the Canadian boreal forest transect case study.Clim. Change74, 223–251 (2006).
Michalzik, B., Kalbitz, K., Park, J. H., Solinger, S. & Matzner, E. Fluxes and concentrations of dissolved organic carbon and nitrogen – a synthesis for temperate forests.Biogeochemistry52, 173–205 (2001).
Pastore, M. A., Hobbie, S. E. & Reich, P. B. Sensitivity of grassland carbon pools to plant diversity, elevated CO2, and soil nitrogen addition over 19 years.Proc. Natl Acad. Sci. USA118, e2016965118 (2021).
Yemshanov, D., McKenney, D. W. & Pedlar, J. H. Mapping forest composition from the Canadian National Forest Inventory and land cover classification maps.Environ. Monit. Assess.184, 4655–4669 (2012).
Baeten, L. et al. Identifying the tree species compositions that maximize ecosystem functioning in European forests.J. Appl. Ecol.56, 733–744 (2019).
Moles, A. T. et al. Global patterns in plant height.J. Ecol.97, 923–932 (2009).
Dyk, A. Forest composition across Canada.Canadian Forest Servicehttps://cfs.nrcan.gc.ca/publications?id=35724(2014).
Butler, E. E. et al. Increasing functional diversity in a global land surface model illustrates uncertainties related to parameter simplification.J. Geophys. Res. Biogeosci.127, e2021JG006606 (2022).
Mason, R. E. et al. Evidence, causes, and consequences of declining nitrogen availability in terrestrial ecosystems.Science376, eabh3767 (2022).
Canadian Forest Inventory Committee.Canada’s National Forest Inventory – Design Overview. Version 3.2(Canadian Forest Service, 2004).
Lambert, M. C., Ung, C. H. & Raulier, F. Canadian national tree aboveground biomass equations.Can. J. For. Res.35, 1996–2018 (2005).
Chen, H. Y. H. & Klinka, K. Aboveground productivity of western hemlock and western redcedar mixed-species stands in southern coastal British Columbia.For. Ecol. Manag.184, 55–64 (2003).
British Columbia Ministry of Forests and Range and British Columbia Ministry of Environment.Field Manual for Describing Terrestrial Ecosystems2nd edn (Province of British Columbia, 2010).
Gillis, M. D., Omule, A. Y. & Brierley, T. Monitoring Canada’s forests: the National Forest Inventory.For. Chron.81, 214–221 (2005).
Pearson, T. R. H., Brown, S. L. & Birdsey, R. A.Measurement Guidelines for the Sequestration of Forest Carbon(U.S. Department of Agriculture, Forest Service, Northern Research Station, 2007).
Pielou, E. C.An Introduction to Mathematical Ecology(Wiley, 1969).
Reich, P. B. The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto.J. Ecol.102, 275–301 (2014).
Kunstler, G. et al. Plant functional traits have globally consistent effects on competition.Nature529, 204–207 (2016).
Hisano, M. & Chen, H. Y. H. Spatial variation in climate modifies effects of functional diversity on biomass dynamics in natural forests across Canada.Glob. Ecol. Biogeogr.29, 682–695 (2020).
Reich, P. B., Walters, M. B. & Ellsworth, D. S. From tropics to tundra: global convergence in plant functioning.Proc. Natl Acad. Sci. USA94, 13730–13734 (1997).
Wright, I. J. et al. The worldwide leaf economics spectrum.Nature428, 821–827 (2004).
Kattge, J. et al. TRY – a global database of plant traits.Glob. Change Biol.17, 2905–2935 (2011).
Laliberte, E. & Legendre, P. A distance-based framework for measuring functional diversity from multiple traits.Ecology91, 299–305 (2010).
Ruiz-Benito, P. et al. Climate- and successional-related changes in functional composition of European forests are strongly driven by tree mortality.Glob. Change Biol.23, 4162–4176 (2017).
Hisano, M., Ryo, M., Chen, X. & Chen, H. Y. H. Rapid functional shifts across high latitude forests over the last 65 years.Glob. Change Biol.27, 3846–3858 (2021).
Diaz, S. et al. The global spectrum of plant form and function.Nature529, 167–173 (2016).
Zeugin, F., Potvin, C., Jansa, J. & Scherer-Lorenzen, M. Is tree diversity an important driver for phosphorus and nitrogen acquisition of a young tropical plantation?For. Ecol. Manag.260, 1424–1433 (2010).
Régnière, J., St-Amant, R. & Béchard, A.BioSIM 10 User’s manual.Report No. LAU-X-137E (Natural Resources Canada, Laurentian Forestry Centre, 2014).
Hogg, E. H. Temporal scaling of moisture and the forest-grassland boundary in western Canada.Agr. For. Meteorol.84, 115–122 (1997).
Senici, D., Chen, H. Y. H., Bergeron, Y. & Cyr, D. Spatiotemporal variations of fire frequency in central boreal forest.Ecosystems13, 1227–1238 (2010).
Rosseel, Y. lavaan: an R package for structural equation modeling.J. Stat. Softw.48, 1–36 (2012).
Oberski, D. lavaan.survey: an R package for complex survey analysis of structural equation models.J. Stat. Softw.571-27(2014)。
Kenny, D. A., Kaniskan, B. & McCoach, D. B. The performance of RMSEA in models with small degrees of freedom.Sociol. Methods Res.44, 486–507 (2015).
R Core Team.R: A Language and Environment for Statistical Computing(R统计的基础istical Computing, 2022).
Acknowledgements
We thank Natural Resources Canada, Canadian Forest Service for sharing data from the National Forest Inventory database and the Discovery Grants programme (grant no. RGPIN-2018-05700 to S.X.C.) of the Natural Sciences and Engineering Research Council of Canada (NSERC) for supporting this research. H.Y.H.C. acknowledges the support from NSERC (RGPIN-2019–05109 and STPGP428641) and the Canada Foundation for Innovation and Ontario Research Fund (CFI36014). X.C. wishes to thank NSERC and the Government of Canada for a Banting Postdoctoral Fellowship and P.B.R. acknowledges support by the U.S. National Science Foundation Biological Integration Institutes grant no. NSF-DBI-2021898.
Author information
Authors and Affiliations
Contributions
X.C., P.B.R., H.Y.H.C. and S.X.C. were responsible for the conception and design of the project. X.C. and A.R.T. compiled data. X.C. analysed the data and wrote the first draft of the manuscript. X.C., A.R.T., P.B.R., M.H., H.Y.H.C. and S.X.C. contributed to reviewing and editing. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Naturethanks the anonymous reviewers for their contribution to the peer review of this work.Peer reviewer reportsare available.
Additional information
Publisher’s noteSpringer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 The result of PCA showing permanent sampling plots and each functional identity.
CWM, community-weighted mean of trait value; CWMNmass, CWM of nitrogen content per leaf mass; CWMPmass, CWM of phosphorus content per leaf mass; CWMSLA, CWM of specific leaf area; CWMWD, CWM of wood density; CWMMH, CWM of maximum height. The first axis (PC1) represents traits associated with acquisitive versus conservative strategies, whereas the second axis (PC2) refers to traits associated with wood density (WD) versus the maximum height (MH) of trees.
Extended Data Fig. 2 The distributions of 406 ground plots from the Canadian NFI with climate and plant community characteristics information.
a, Long-term averages of mean annual temperature (MAT).b, Long-term averages of mean annual climate moisture index (CMI).c, Species richness.d, Species evenness.e, Functional diversity (FDis).f,g, CWM of trait value (CWMPC1, CWMPC2).h, Schematic diagram of the NFI ground plot.
Extended Data Fig. 3 The bivariate relationships between decadal changes in soil C and N stocks in the organic horizon (ΔSoil COrganicstock and ΔSoil NOrganicstock) and explanatory variables (n = 361) for all proposed causal paths in the structural equation model.
MAT, the long-term average of mean annual temperature; CMI, the long-term average of mean annual climate moisture index; FDis, functional diversity; Horizon thickness, initial organic horizon thickness; ΔThickness, decadal organic horizon soil thickness change. Higher CWMPC1值指示性状与贪婪有关strategy, whereas lower values indicate conservative strategy. Higher CWMPC2values indicate traits associated with lower tree maximum height (see Extended Data Fig.1).
Extended Data Fig. 4 The bivariate relationships between decadal changes in soil thickness in the organic horizon (ΔThickness) and explanatory variables (n = 361) for all proposed causal paths in the structural equation model.
All fitted regressions are significant atP < 0.05. MAT, the long-term average of mean annual temperature; CMI, the long-term average of mean annual climate moisture index; FDis, functional diversity; Horizon thickness, initial organic horizon thickness. Higher CWMPC1值指示性状与贪婪有关strategy, whereas lower values indicate conservative strategy. Higher CWMPC2values indicate traits associated with lower tree maximum height (see Extended Data Fig.1).
Extended Data Fig. 5 The bivariate relationships between decadal changes in soil C and N stocks in the mineral horizon (ΔSoil CMineralstock and ΔSoil NMineralstock) and explanatory variables (n = 245) for all proposed causal paths in the structural equation model.
All fitted regressions are significant atP < 0.05. MAT, the long-term average of mean annual temperature; CMI, the long-term average of mean annual climate moisture index; FDis, functional diversity. Higher CWMPC1值指示性状与贪婪有关strategy, whereas lower values indicate conservative strategy. Higher CWMPC2values indicate traits associated with lower tree maximum height (see Extended Data Fig.1).
Extended Data Fig. 6 The bivariate relationships between decadal aboveground primary productivity and explanatory variables for all proposed causal paths in the structural equation model.
All fitted regressions are significant atP < 0.05. MAT, the long-term average of mean annual temperature; CMI, the long-term average of mean annual climate moisture index; FDis, functional diversity. Higher CWMPC1值指示性状与贪婪有关strategy, whereas lower values indicate conservative strategy. Higher CWMPC2values indicate traits associated with lower tree maximum height (see Extended Data Fig.1).
Extended Data Fig. 7 The bivariate relationships between decadal relative changes in soil C and N stocks in the mineral horizon and mineral horizon soil C and N content, respectively.
All fitted regressions are significant atP < 0.05. Dotted vertical line represents the soil C or N content when relative changes in soil C and N stocks began to shift from positive to negative.
Extended Data Fig. 8 Structural equation models showing the effects of tree diversity, alternative climatic factors and soil conditions on decadal changes in soil C and N stocks.
a,b, Path diagrams of factors influencing changes in soil C and N stocks in the organic horizon (n = 361).b,dPath diagrams of factors influencing changes in soil C and N stocks in the mineral horizon (n = 245). Numbers adjacent to arrows are standardized path coefficients, analogous to relative regression weights. Solid and dashed arrows represent positive and negative relationships, respectively. Different colours represent different types of explanatory variable (see Fig.1). Only significant pathways are shown (P < 0.05). The goodness-of-fit statistics for panelsa–dare: GFI = 0.988, SRMR = 0.032,P = 0.249; GFI = 0.991, SRMR = 0.029,P = 0.477; GFI = 0.987, SRMR = 0.033,P = 0.367; and GFI = 0.986, SRMR = 0.038,P = 0.391, respectively, indicating close model-data fit. ΔSoil COrganicand ΔSoil NOrganicrepresent decadal changes in soil C and N stocks of the organic soil horizons, respectively. ΔSoil CMineraland ΔSoil NMineralrepresent decadal changes in soil C and N stocks of the mineral soil horizons, respectively. GDD, mean annual growing degree-days; AGP, mean annual precipitation at the growing season; FDis, functional diversity; ΔThickness, decadal changes in soil organic horizon thickness; CWMPC2community-weighted意味着特征值;地平线thickness, initial organic horizon thickness. Higher CWMPC2values indicate traits associated with lower tree maximum height (see Extended Data Fig.1).
Supplementary information
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, X., Taylor, A.R., Reich, P.B.et al.Tree diversity increases decadal forest soil carbon and nitrogen accrual.Nature(2023). https://doi.org/10.1038/s41586-023-05941-9
Received:
Accepted:
Published:
DOI:https://doi.org/10.1038/s41586-023-05941-9
Comments
By submitting a comment you agree to abide by ourTermsandCommunity Guidelines.If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
