不对称的猫alysis
不对称的猫alysis is a type of catalysis in which a chiral catalyst directs the formation of a chiral compound such that formation of one particular stereoisomer is favoured. Since the catalyst is not consumed in this process it may be used in a substoichiometric quantity – potentially improving efficiency and avoiding waste.

 Atroposelectivity with two catalysts
Atroposelectivity with two catalysts Stereoselective alkyl–alkyl cross-coupling
Stereoselective alkyl–alkyl cross-coupling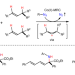 Metalloradical approach for concurrent control in intermolecular radical allylic C−H amination
Metalloradical approach for concurrent control in intermolecular radical allylic C−H amination Site- and enantioselective cross-coupling of saturatedN-heterocycles with carboxylic acids by cooperative Ni/photoredox catalysis
Site- and enantioselective cross-coupling of saturatedN-heterocycles with carboxylic acids by cooperative Ni/photoredox catalysis Synthesis of axially chiral alkenylboronates through combined copper- and palladium-catalysed atroposelective arylboration of alkynes
Synthesis of axially chiral alkenylboronates through combined copper- and palladium-catalysed atroposelective arylboration of alkynes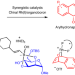 A synergistic Rh(I)/organoboron-catalysed site-selective carbohydrate functionalization that involves multiple stereocontrol
A synergistic Rh(I)/organoboron-catalysed site-selective carbohydrate functionalization that involves multiple stereocontrol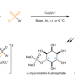 Cu-catalysed enantioselective radical heteroatomic S–O cross-coupling
Cu-catalysed enantioselective radical heteroatomic S–O cross-coupling Enantio- and diastereoselective construction of vicinal C(sp3) centres via nickel-catalysed hydroalkylation of alkenes
Enantio- and diastereoselective construction of vicinal C(sp3) centres via nickel-catalysed hydroalkylation of alkenes Radical catalysis breaks and makes bonds
Radical catalysis breaks and makes bonds Copper for alkynylallylic substitution
Copper for alkynylallylic substitution Kinetic resolution of planar chiral metallocenes
Kinetic resolution of planar chiral metallocenes Synthesis of cycloheptanoids through asymmetric catalytic (4 + 3) cycloadditions
Synthesis of cycloheptanoids through asymmetric catalytic (4 + 3) cycloadditions